Description
In 1997, the Food and Drug Administration (FDA) approved the use of a radiopharmaceutical, technetium-99m sestamibi (Miraluma) for scintimammography, which used a conventional gamma camera to image the breast. Technetium-99m sestamibi, showed a high propensity to accumulate in breast tumors, which allowed for “functional imaging” not affected by anatomic characteristics such as breast density or surgical distortion. There are two mechanisms by which Technetium-99m sestamibi accumulates in breast tumors. The first is that it binds preferentially to the mitochondria in cells; since cancers have a high density of mitochondria (which is a marker for proliferative activity) they accumulate more technetium-99m sestamibi than the adjacent normal tissue.1 Secondly, breast tumors produce significant neoangiogenesis that enables increased uptake of the radiopharmaceutical.2
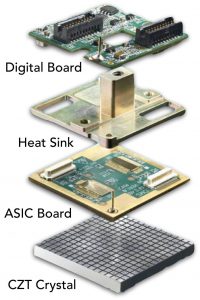
Although scintimammography with conventional gamma cameras showed good sensitivity and specificity for larger tumors, its low sensitivity for tumors smaller than 15 mm did not meet prevailing standards for breast imaging.3, 4 Improvements in gamma camera technology and optimized image acquisition parameters allowed for higher sensitivity for detecting subcentimeter lesions, and ushered in the upgraded technology to now be referred to as MBI. Two types of gamma cameras are currently available: 1) multicrystal array detectors using sodium iodide, and 2) cadmium zinc telluride (CZT) direct conversion detectors.
The use of cadmium zinc telluride (CZT) direct conversion detectors showcase a technology upgrade in nuclear medicine where photon energy released by a radiopharmaceutical, such as technetium-99m sestamibi, is directly converted into electric signals allowing for accurate identification of event location and energy. With computer and software technology, complex calculations can be performed very quickly to convert the detected radiation into images and information that is useful for radiologists.
LOW BREAST RADIATION RISK COMPARED TO MAMMOGRAPHY
Optimizations in detector design, patient preparation, and radiopharmaceutical delivery have reduced the administered activity from 740–1,100MBq (20–30 mCi) to the current off-label standard of 240–300MBq (6.5–8 mCi).5, 6 The average absorbed radiation dose to the breast from 300MBq (8 mCi) of 99mTc-sestamibi is estimated to be 1.1mGy, compared with 3.0–4.5mGy that is associated with mammography and breast tomosynthesis (3D mammography).7
Since 99mTc-sestamibi is systemically distributed, tissues outside the breast receive the largest radiation dose. The estimated effective (whole-body) dose for 300MBq (8 mCi) of 99mTc-sestamibi is 2.1–2.6 mSv, which is at, or lower than, annual natural background levels (~3 mSv).8 For reference, the effective dose of chest CT can approach 6 mSv.9 Tissues with the highest exposures include the colon (7.1mGy), urinary bladder (3.2mGy), and gallbladder (11.5mGy).10 Various organizations assessing radiation risk and radiation protection (Health Physics Society, American Association of Physicists in Medicine, International Organization for Medical Physics, and United Nations Scientific Committee on the Effects of Atomic Radiation) state that risks from radiation doses of less than 100 mSv are not significantly different from zero.8 Thus, current MBI radiation exposure is deemed to pose negligible risk to the patient, with minimal theoretic risk of inducing cancer in any of these organs.8 The administered activity of 99mTc-sestamibi for MBI continues to decrease with technologic advancement. Tao et al. showed that new image-processing algorithms maintain lesion conspicuity with a simulated half-dose (150MBq [4 mCi]) injection.11 Furthermore, continued advances in CZT module design will improve sensitivity and should allow further dose reduction.